Publish Date: July 28, 2022
mPRAGATI, a One Stop MedTech Technology Development Facility at IIT Delhi Under ICMR-Medical Device and Diagnostics Mission Secretariat, Unveiled
Share this on

New Delhi: The Indian Council of Medical Research (ICMR) and the Department of Health Research (DHR) formally unveiled MedTech Product Development Acceleration Gateway of India (mPRAGATI), a National Center for medical technology development under the ICMR-Medical Device and Diagnostics Mission Secretariat at IIT Delhi, on June 24, 2022.
mPRAGATI at IIT Delhi, which is sponsored by the ICMR and the DHR under PM-Ayushman Bharat Health Infrastructure Mission (PM-ABHIM), will serve as an ISO certified, C-GMP compliant medical device manufacturing and testing facility to translate medical products from TRL 3 (proof-of-concept) to TRL 7 (ready for clinical evaluation). The facility is currently developing technologies for disease diagnosis, implantable devices for orthopedic, ocular and cardiac needs.
In addition to its formal activities, the facility will also conduct periodic boot camps, manpower training sessions for students, faculty and start-ups to foster the medical device development ecosystem. The facility will bring in technical consultants to fine tune designs and products to keep them at par with industry standard.
This new initiative will bring about a major change in the biomedical ecosystem and help researchers, doctors, entrepreneurs, and academicians across India to effectively take their idea to market. Moreover, it will create a pipeline of advanced technologies and products in line with PM-ABHIM initiatives and will be monitored by the NITI Aayog.
mPRAGATI is led by Prof. Dinesh Kalyanasundaram and Prof. Ravikrishnan Elangovan from IIT Delhi.
To know more about the Centre, please email- dinesh@cbme.iitd.ac.in / dineshk.iitdelhi@gmail.com
**********
Other News

Indian Navy’s Directorate of Naval Architecture Signs MoU with IIT Delhi for Crew Centred Aspects of Warship Design
Read More
Online CEP programme in "Executive Management Programme in Entrepreneurship Development ( Batch 4)"
Read More
IIT Delhi and Samco Inc., Japan, Join Hands to Boost Semiconductor and High-Efficiency Silicon Solar Cell Research
Read More
Online CEP programme in "Executive Programme for Advanced Product Management ( Batch 04 )"
Read More
Online CEP programme in "Executive Programme in Semiconductor Manufacturing and Technology (Batch 2)"
Read More
IHFC, the TIH of IIT Delhi, Showcases 25 Cutting-Edge Technologies to Mark its 5th Anniversary
Read More
Adi Karmayogi Student Chapters Launched at IITs, IIMs, AIIMS, NITs and Top Institutions – Empowering Tribal Youth as Leaders, Innovators, and Change-Makers
Read More
Online CEP programme in "Certificate Programme in Data Science and Machine Learning ( Batch - 11)"
Read More
Online CEP programme in "Certification in Quantum Computing and Machine Learning (Batch 07)"
Read More
Shri Dharmendra Pradhan, Minister of Education, Inaugurates Key Programs and Startup Incubator at IIT Delhi - Abu Dhabi, Reinforcing a Landmark Bilateral Partnership
Read More
SAI NCSSR and IIT Delhi Sign MoU to Give Impetus to Wider Use of Sports Science and Innovation in India
Read More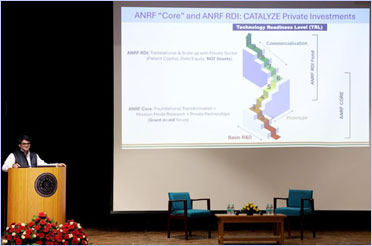
CEO of ANRF Delivers an Insightful Institute Lecture titled “ANRF Vision: Catalyzing India’s Rise as a Research and Innovation Powerhouse” at IIT Delhi
Read More
IIT Delhi Inaugurates Biosafety Level 3 Research Facility to Foster Research Involving Highly Infectious Pathogens
Read More
IIT Delhi and Wadhwani Foundation Inaugurate Centre of Excellence in Precision & Personalized Healthcare under Wadhwani Innovation Network
Read More
Union Minister of Textiles Launches Report on Handloom Carbon Footprint Assessment Prepared by IIT Delhi and DC (Handlooms)
Read More
Online CEP programme in "Certificate Programme for Future Tech Leaders AI & Industry 5.0 (Batch 1)"
Read More
56th Convocation: 2764 IIT Delhi Students Awarded Degrees and Diplomas; Chief Guest Dr. Tessy Thomas, the Missile Woman of India, Inspires Graduating Students
Read More
IIT Delhi to Hold 56th Convocation on August 2, 2025; the Missile Woman of India, Dr. Tessy Thomas, to be the Chief Guest
Read More
Online CEP programme in "Artificial Intelligence and Machine Learning for Industry (Batch 6)".
Read More
Online PG Diploma programme in "Online PG Diploma In Healthcare Product Development And Management".
Read More
Online PG Diploma programme in "Online PG Diploma In Advanced Communication Engineering With Quantum And AI Integration".
Read More
Online CEP programme in "Executive Programme In Operations Management And Analytics (Batch - 6 )".
Read More
Online CEP programme in "Executive Programme In Generative AI And Next Gen Digital Engineering (Batch 1 )".
Read More
Online CEP programme in "Advanced Certificate Programme In AI, ML And DL ( Formerly Known As Certificate Programme In Machine Learning And Deep Learning )".
Read More
Online CEP programme in "Applied Data Science And Artificial Intelligence: From Fundamentals To Deployment - Batch 02".
Read More
Online CEP programme in "Executive Programme In Augmented Reality & Virtual Reality (Batch - 2 )".
Read More
Workshop on ‘Future Ready Energy Education: Opportunities and Challenges’ Held at IIT Delhi
Read More
Change Makers 2025 Summer Bootcamp Successfully Culminates at IIT Delhi with High School and 1st Year UG Students Presenting Promising Innovative Solutions to Environmental Challenges
Read More
QS World University Rankings 2026: IIT Delhi Features in List of Top 125 World Institutions; Emerges as No. 1 Educational Institute in India
Read More
IIT Delhi and LG Electronics Partner to Drive Innovation in Sustainable Home Appliance Technologies
Read More
World Environment Day 2025 Celebrated at IIT Delhi; Institute Highlights Need for Collective Action
Read More
IIT Delhi to Organise Open House in Delhi, Mumbai and Bengaluru for JEE Advanced 2025 Qualified Candidates
Read More
IIT Delhi launches new undergraduate programme BS in Chemistry for JEE (Advanced) 2025 qualified candidates
Read More
Central Electricity Regulatory Commission, IIT Delhi and Grid Controller of India sign MoU to Establish Centre of Excellence for Regulatory Affairs in Power Sector
Read More
Provisional Waiting List of Candidates for the Ph.D. Program in Materials Science and Engineering
Read More
HORIBA India Join Hands with IIT Delhi to Support Three Technical Development Research Projects
Read More
Provisional *List of Selected Candidates for Ph.D. in Materials Science and Engineering, *Subject to Document Verification by the Academic Section DMSE
Read More
IIT Delhi Hosts ‘Manasvi: STEM Mentorship Program for High School Girls’ – Empowering Young Girls with Exposure and Opportunities to Become Future-Ready for STEM
Read More
IIT Delhi Debuts Online Post Graduate Diplomas to Empower Next-Gen Technological Leadership
Read More
Manufacturing Innovation Show: Agricultural Machineries, On-road Power Generation Device Among Several Prototypes Developed by 1st Year IIT Delhi UG Students
Read More
IIT Delhi Establishes a Chair on Applied AI for Sustainable Systems in Collaboration with R Systems
Read More
Fuelling a Sustainable Future: IIT Delhi-Abu Dhabi Announces Second Intake for Post-Graduate Energy & Sustainability Programs
Read More
IIT Delhi Successfully Conducts CAIC Student Elections Through E-Voting, Eyes Scaling to Larger Platforms
Read More
Shortlisted candidates list & shortlisting Criteria for PhD admission in Textile department
Read More
Shortlisted candidates list & shortlisting Criteria for MTech admission in Textile department
Read More
IIT Delhi, Central Water Commission Sign MoU for Water Resources Management Through Data Science, AI and Machine Learning
Read More
IIT Delhi Celebrates the Legacy of Dr. B.R. Ambedkar with “Jai Bhim Saptah” – A Week of Reflection, Dialogue, and Action
Read More
Placement 2024-25: UG Students at IIT Delhi Secure 850 Unique Offers to Date, Highest in Last Three Years
Read More
Bhagwan Birsa Munda Cell at IIT Delhi Hosts Tribal Students and Youth for an Exposure Visit
Read More
Empowering the Future: Through NURTURE Program IHFC (TIH of IIT Delhi) Trains Over 50,000 SC/ST Students in Cutting-Edge Technologies
Read More
Online CEP programme in "Certificate Programme in Machine Learning and Deep Learning (Batch 6)."
Read More
Online CEP programme in "Advance Programme in Electric Vehicle (EV) Technology (Batch 8)."
Read More
Quantum Materials & Devices (QMD) Hub at IIT Delhi Successfully Hosts its Grand Inception & Strategy Summit
Read More
Online CEP programme in "Certificate Programme in Data Science & Machine Learning(Batch 10)"
Read More
Online CEP programme in "Artificial Intelligence and Machine Learning for Industry (Batch 05)"
Read More
IIT Delhi Invites Applications from High Schoolers and 1st Year UG Students for Change Makers 2025 Summer Bootcamp
Read More
JAM Admission Portal JOAPS for Various Master’s Programs in IITs to Open from March 26, 2025
Read More
IIT Delhi organises ‘Anveshan 2025’ as a part of the Institute’s initiative of “Experience IITD”
Read More
International Happiness Day Celebration marks the inauguration of Rekhi Mind Lab at IIT Delhi to promote Happiness Studies
Read More
Online CEP programme in "Certificate Programme in Hybrid Electric Vehicles (HEVs) Design (Batch 2) "
Read More
Online CEP programme in "Advanced Certification in Data Science and Decision Science (Batch 5) "
Read More
New Zealand Prime Minister Strengthens Academic Ties with India, Announces Scholarships at IIT Delhi
Read More
QS World University Rankings by Subject 2025: IIT Delhi Features Among Top 30 World Institutions in Engineering & Technology Category
Read More
IIT Delhi to Organise an Insightful Event, “Anveshan: Innovation and Exploration Across Disciplines”, for Undergraduate and Postgraduate Students, Showcasing Curriculum, Research, and Campus Life
Read More
SANGAM, An Inclusive Intercollege Sports Event for Students with Disabilities, Held at IIT Delhi
Read More
Inaugural Chidambaram Memorial Lecture held at IIT Delhi on National Science Day; Prof Abhay Karandikar, DST Secretary, Delivered the Inaugural Lecture
Read More
Bhagwan Birsa Munda Cell at IIT Delhi Hosts an Exposure Visit for 200 Tribal Students from Five States
Read More
Opportunity to work on the UQ - IITD Research Academy and Weir IP Group Ltd., Scotland supported project
Read More
IIT Delhi Opens Its Doors to Teachers from Technical Institutions in Haryana for Skill Enhancement and Research-Driven Learning
Read More
IIT Delhi, IndiGo Sign MoU to Drive Innovation and Create Impactful Solutions for Airline Industry
Read More
College Youth Ideathon 2025: An initiative to Fuel Innovation and Entrepreneurship Among India’s Youth Launched by IIT Delhi, MEPSC, and ThinkStartup
Read More
IIT Delhi and University of Exeter, UK, Host Joint Symposium on Sports Healthcare Science and Engineering
Read More
2nd Transformative Leadership in STEMM (TLS) Workshop for Ph.D. Scholars from SC/ST Community Held at IIT Delhi
Read More
IHFC Collaborates with 10 Leading Institutions Across India to Launch Transformative Co-Innovation Centres (CiC) in Deep Tech, AI and Robotics
Read More
150 High School Students Attend Inaugural Lecture Under “Sci-Tech Spins” Lecture Series 2025 at IIT Delhi
Read More
Online CEP programme "Certificate Programme in Applied Data Science & Artificial Intelligence: From Fundamentals to Deployment"
Read More
CEP programme "One-week Training Programme on Construction Quality Control and Management"
Read More
Swami Vivekananda Jayanti at IIT Delhi: Experts talk on Vedanta, Leadership, and India's Educational Legacy
Read More
NISHAAN 2025, a Cultural Event Celebrating Diversity and Talent of Students with Disabilities, Organised at IIT Delhi
Read More
Third Batch of IIT Delhi’s STEM Mentorship Program for High School Girls Successfully Concludes
Read More
IIT Delhi Hosts Mr. Amandeep Singh Gill, UN Under-Secretary-General for Digital and Emerging Technologies and Secretary-General's Envoy on Technology, For a Discussion on AI Governance
Read More
Jake Sullivan, USA’s National Security Advisor, Delivers a Talk on 'The United States and India: Building a Shared Future' at IIT Delhi
Read More
TRIP Centre, IIT Delhi, organizes a roundtable discussion on “Gendered Resilience in Transport: Enhancing Adaptation to Extreme Heat in Delhi”
Read More
DRDO Industry Academia-Center of Excellence (DIA-CoE) at IIT Delhi Transfers Technology of ABHED, a Light Weight Bulletproof Jacket, to three Indian Industries
Read More
CEP programme on "Certificate Programme in Industrial Polymer Production: Combined Chemistry and Chemical Engineering Approaches"
Read More
IIT Delhi Students Are Choosing Diverse Career Paths, Shows the Graduation Exit Survey 2024
Read More
DFPD Launches ‘Anna Chakra’ PDS Supply Chain Optimization Tool Developed by PSL, IIT Delhi
Read More
IIT Delhi Leads Landmark Collaboration with Hyundai Motor Group to Advance Battery and Electrification Research
Read More
3rd online CEP programme on "Executive Programme in Healthcare Entrepreneurship and Management"
Read More
Bhagwan Birsa Munda Cell at IIT Delhi Hosts Commemoratives Events on Janjatiya Gaurav Diwas
Read More
AIIMS New Delhi, IIT Delhi and UCL Announce Trilateral Partnership to Scale Up MedTech Innovation
Read More
Call for Applications: Transformative Leadership in STEMM (TLS) Workshop for Advanced PhD Scholars from SC/ST community.
Read More
IIT Delhi and Centre for Railway Information Systems Partner to Transform Indian Railways Through Cutting-Edge Research
Read More
AI to Empower, Not Threaten: Meta's Chief AI Scientist Yann LeCun Calls for New Model Architectures at IIT Delhi
Read More
IIT Delhi Launches MS (Research) Program in ‘Healthcare Technology’ for Medical and Allied Clinical Professionals
Read More
Workshops Organised for Parents and School Kids to Raise Awareness and Co-Create Possible Solutions to Beat the Heat
Read More
Joint Report by IIT Delhi and IEA: "Clean Energy Innovation Policies in Emerging and Developing Economies"
Read More
PGDEx-VLFM Batch 07 Students Meet IIT Delhi Director; Witness an Inspiring Interactive Session
Read More
IIT Delhi, IAF Join Hands for AI-powered Research on Technical Textiles; to Focus on Parachute and Other Safety Equipment
Read More
4th online CEP programme "Executive Management Programme in Supply Chain & Operations Analytics"
Read More
India's first-of-its-kind DST Supported CO2-to-Methanol Pilot Plant Project to be Implemented by IIT Delhi and Thermax Ltd. in PPP Mode
Read More
Major Milestones for IIT Delhi as His Highness Sheikh Khaled bin Mohamed Visits Abu Dhabi Campus
Read More
IIT Delhi and Systra Group Sign MoU to Collaborate on Research Advancing Sustainable Development Goals
Read More
Joint Admission Test for Masters (JAM) 2025: Application window opens on 3rd September 2024
Read More
Materials Science and Engineering Dept. at IIT Delhi Hosts Training Program on ‘Polymer Science and Technology’ for IOCL Executives
Read More
Rashtriya Vigyan Puraskar 2024: Prof. Bhim Singh, SERB National Science Chair and Emeritus Professor at IIT Delhi, Honored with the Vigyan Shri Award
Read More
CEP programme "Workshop on Enabling Renewable Energy Integration in Modern Power Systems with Advanced Technologies"
Read More
IIT Delhi Honors Alumni with Prestigious Distinguished Alumni Awards 2024 at 55th Convocation Ceremony
Read More
IIT Delhi to Hold its 55th Convocation on August 10, 2024; Shri. Hari S. Bhartia, Founder and Co-Chairman, Jubilant Bhartia Group to be Chief Guest
Read More
Important Information for PhD students regarding seating of Guests in Dogra Hall in 1st Phase
Read More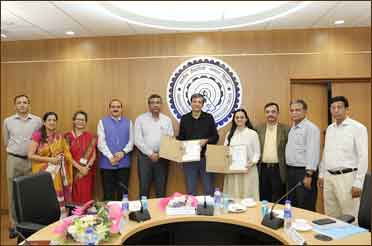
IIT Delhi Develops Two Pioneering Healthcare Technologies; Successfully Transfers them to Industry
Read More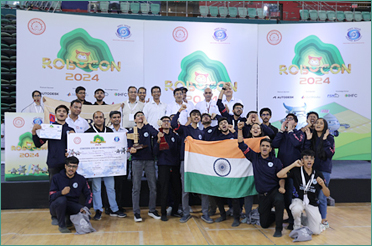
DD Robocon India 2024: Nirma University from Gujarat Wins Robotics Competition; Maharashtra’s Pimpri Chinchwad College of Engineering Secures Runner-up Position
Read More
Initiative for Gender Equity and Sensitisation (IGES) at IIT Delhi to Organise Wikipedia Edit-a-Thon on July 5
Read More
Bridging the Gap: IIT Delhi’s Aab Prahari App Enables Citizens to Report Real-Time Flooding Incidents and Help Civic Agencies During Monsoon
Read More
An Open House for All JEE (Advanced) 2024-Qualified Candidates Aspiring to Join IIT Delhi’s Hauz Khas Campus in India or Abu Dhabi Campus in UAE to be Organised on June 15, 2024
Read More
Students Clearing JEE Advanced 2024 can apply for Admissions to IIT Delhi-Abu Dhabi Campus
Read More
UQ-IITD Research Academy - Promotion of the Call for Collaborative Research grant applications
Read More
Entrepreneurship Development Cell at IIT Delhi Collaborates with Alumni to Conduct an Entrepreneurship Course for Students on Building Successful Startups from Scratch
Read More
IIT Delhi’s Technology Innovation Hub IHFC, ITU Collaborate to Organise ‘Robotics for Good Youth Challenge’
Read More
2nd online CEP programme on "Executive Programme in Product Innovation and Design Thinking for Business Growth".
Read More
SUMMER RESEARCH FELLOWSHIP PROGRAMME 2024 FOR M.TECH. /M.E. STUDENTS AT INDIAN INSTITUTE OF TECHNOLOGY DELHI
Read More
QS World University Rankings by Subject 2024: IIT Delhi Ranked Among the Top 100 World Institutions in 08 Specific Subject Areas; Features Among Top 50 in Engineering and Technology
Read More
International Women's Day: Talk titled "Rise to Shine: Embracing Challenges for Success” Organised
Read More
Antara Senior Care Signs MoU with IIT Delhi to Design Innovative Mobility-aid Solutions for Seniors
Read More
Israel Aerospace Industries and IIT Delhi Sign CSR Agreement to Collaborate on Applied Research
Read More
IIT Delhi’s Department of Humanities and Social Sciences to Offer a New Academic Program ‘M.A. in Culture, Society, Thought’
Read More
Completion of Sequencing of 10000 Genomes of Indian Population: IIT Delhi Scientists Make Significant Contribution
Read More
Bill Gates Inspires IIT Delhi Students, Encourages Them to Apply Their Skills to Global Challenges
Read More
03rd online CEP programme titled "Executive Management Programme in Advanced Strategic Management"
Read More
LC3-TRC Africa Project Mentored by IIT Delhi Researchers Inaugurated at Kenya’s Meru University of Science and Technology
Read More
R Systems International Ltd. and IIT Delhi Partner to Set Up a Centre of Excellence on Applied AI for Sustainable Systems
Read More
Transformative Leadership in STEMM (TLS) Workshop for Ph.D. Scholars from SC/ST Community Held at IIT Delhi
Read More
IIT Delhi Launches a New Academic Program ‘M.Sc. in Biological Sciences’; Admissions Through JAM
Read More
GRIP: IIT Delhi Students Visit Uttarakhand Villages; Will Develop Tech Solutions to Address Issues Faced by Local Population
Read More
National Service Scheme (NSS) of IIT Delhi Launches an In-house-developed Mobile App, Making Volunteering Much More Accessible
Read More
Call for Applications: Transformative Leadership in STEMM (TLS) Workshop for Advanced PhD Scholars from SC/ST community.
Read More
Request for Proposal: Empanelment of Service providers for the Online CEP Programmes at IIT Delhi
Read More
2nd online CEP programme titled "Executive Programme in Healthcare Entrepreneurship and Management"
Read More
Consultative Brainstorming Meeting: ‘DST's Roadmap Towards India’s Net Zero Targets Through Carbon Capture, Utilization, and Storage’
Read More
04th online CEP programme titled "Certification in Quantum Computing and Machine Learning"
Read More
IIT Delhi Faculty Combines Technology with Art Forms to Offer a Holistic Educational Experience to Materials Engineering Students
Read More
Placement Season Begins at IIT Delhi- Students Receive 480 Job Offers By the End of December 1
Read More
Over 30 High School Girls Successfully Complete Second STEM Mentorship Program Launched by IIT Delhi
Read More
BioTEX-2023 : Organized by the CBME, Indian Institute of Technology (IIT) Delhi at New Delhi from 27th November-1st December 2023
Read More
Shortlisting Criteria and List of candidates shortlisted for interview for the MSR Program in the Department of Materials Science and Engineering for the session 2023-24, II Semester
Read More
Short-listing criteria for admission to Ph.D. in Materials Science and Engineering for the year 2023-2024 (2nd Semester)
Read More
IIT DELHI ANNOUNCES M. TECH. IN ENERGY TRANSITION AND SUSTAINABILITY FOR THE ABU DHABI CAMPUS: Last date extended to November 30, 2023
Read More
3rd online CEP programme titled "Advanced Certification in Data Science and Decision Science"
Read More
online CEP programme titled "Hands-on Training on "Semiconductor Device Technology: Fabrication and Characterization"
Read More
IIT Delhi Faculty Prof. Shilpi Sharma and Prof. Anurag Singh Rathore Win Tata Transformation Prize; Recognized for their Cutting-Edge Solutions to Food Security and Healthcare
Read More
Hon'ble Vice-President of India, Shri Jagdeep Dhankhar, Visits IIT Delhi and Interacts with Students
Read More
In pictures: Hon'ble Vice-President of India, Shri Jagdeep Dhankhar, Visits IIT Delhi and Interacts with Students
Read More
Tehri Hydro Development Corporation India Limited (THDCIL) and IIT Delhi Ink MoU for Transformative Research and Development Initiatives
Read More
DAKSH Centre of Excellence for Law and Technology at IIT Delhi Launches a Book Titled ‘Technology and Analytics for Law and Justice’
Read More
IIT Delhi and HORIBA India Jointly Hold Tech Symposium on ‘Solutions for Semiconductor Industry’
Read More
In pictures: Birth Anniversary of Mahatma Gandhi and Shri Lal Bahadur Shastri Celebrated at IIT Delhi
Read More
First of Its Kind ‘Medical Cobotics Centre (MCC)’ Inaugurated in New Delhi; to Foster Innovation in Healthcare
Read More
online CEP programme titled "Certificate Programme in Project Management: Theory & Practice"
Read More
IIT Delhi’s Prof. Dipti Ranjan Sahoo Wins Shanti Swarup Bhatnagar Prize for Science and Technology 2022
Read More
IIT Delhi and EXL Enter into MoU to Work Towards Empowering Women Entrepreneurs Through Digital and Financial Literacy
Read More
2nd online CEP programme titled "Executive Management Programme in Advanced Strategic Management"
Read More
First Batch of UQIDAR Students Receive Joint PhD Degree at 54th IIT Delhi Convocation Ceremony
Read More
In pictures: IIT Delhi Celebrates Independence Day; Employees Awarded for Exemplary Contribution to Institute
Read More
IIT Delhi's School of Public Policy Successfully Places its Inaugural Masters in Public Policy Batch
Read More
Workshop on MedTech and Healthcare Ecosystem for Upcoming IIT Delhi - Jhajjar Campus Organised
Read More
2357 Graduating IIT Delhi Students Receive Degrees and Diplomas at the 54th Annual Convocation Ceremony
Read More
IIT Delhi to Hold its 54th Convocation on August 12; Eminent Virologist Dr. Gagandeep Kang to be the Chief Guest
Read More
IIT Delhi Successfully Organizes Open House for JEE (Advanced) 2023 Qualified Female and PwD Candidates
Read More
IIT Delhi Research Scholar Anchal Sharma Makes Research Presentation Before Indian PM and US First Lady at NSF
Read More
IIT Delhi is organising an Open House for JEE (Advanced) 2023 qualified Female and Pwd Candidates on June 24, 2023 (Saturday)
Read More
online CEP programme titled "Applied Data Science using Machine Learning & Artificial Intelligence"
Read More
IIT Delhi Celebrates World Telecommunication Day; India’s G20 Sherpa, Mr. Amitabh Kant, Delivers Annual Bharti Lecture
Read More
RESULT OF SUMMER RESEARCH FELLOWSHIP PROGRAMME -2023 WILL BE DECLARED BY THE SECOND WEEK OF MAY, 2023
Read More
MechAnalyzer Software to Help Engineering Students Learn Concepts of Mechanisms; FITT-IIT Delhi and SVR InfoTech Sign MoU for Sale and Tech Support
Read More
Venus Chair Established at IIT Delhi to Support Teaching and Research & Development in the Area of Fibrous Air Filters
Read More
Deadline of Submission of Online Applications for SRFP - 2023 extended upto 19.04.2023 till 11:59 PM
Read More
2nd online CEP programme titled "Advanced Certification in Data Science and Decision Science (Batch 2) "
Read More
IIT Delhi’s Two-Day Annual Career Fest ‘Pravritti 2023- Expanding Horizons’ to Begin on April
Read More
2nd online CEP programme titled "Executive Programme for Advanced Product Management (Batch 2)"
Read More
online CEP programme titled "Executive Programme in Product Innovation & Design Thinking for Business Growth"
Read More
QS World University Rankings by Subject 2023: IIT Delhi Among the Top 50 Institutions in the World in Engineering & Technology with 48th Rank
Read More
MoS for Education Dr. Subhas Sarkar Inaugurates Unnat Bharat Abhiyan's "UNNATI Mahotsav and Expo" at IIT Delhi
Read More
In pictures- Hon'ble Australian PM Mr. Anthony Albanese's Visit to IIT Delhi on March 10, 2023
Read More
Hon’ble Australian Prime Minister Mr. Anthony Albanese Visits IIT Delhi; Addresses Students
Read More
IIT Delhi’s Three-day Annual Science, Technology and Management Festival “Tryst 2023” Kicks Off
Read More
IIT Delhi Alumnus Alok Aggarwal Endows Chair for Research in ESG (Environmental, Social, and Governance) Area
Read More
IIT Delhi to Develop Smart Monitoring System to Ensure Safety of Persons Working at Height
Read More
65th Foundation Day of IIT Delhi Celebrated; Faculty Research Awards 2022 Presented on Foundation Day
Read More
Mobility for All: Continental with IIT Delhi Develop A Solution for Visually Impaired To Access Public Buses
Read More
27th Inter-IIT Staff Sports Meet: IIT Delhi Wins Overall Championship and General Championship (Men)
Read More
IIT Delhi Placement Drive 2022-23: Students Receive Record Number of Job Offers Up to December 15
Read More
IIT Delhi and University of Helsinki, Finland, Sign MoU for Academic Cooperation; Aims to Contribute to Solving Air Quality and Climate Change Challenges in India
Read More
CRDT, IIT Delhi Organises International Symposium on Circular Economy Solutions for Plastics and Microplastics jointly with University of Eastern Finland (UEF)
Read More
Four Startups led by IIT Delhi Students Win a Grant of Rs 50 lakh Each Under Endowment Nurture Fund Initiative
Read More
Advertisement inviting applications for the post of Director, IIT IIT Kanpur and IIT (ISM) Dhanbad
Read More
IIT Delhi Holds 53rd Annual Convocation Ceremony; 2100 Graduating Students Awarded Degrees and Diplomas
Read More
Rural Technology Action Group (RuTAG) at IIT Delhi Transfers Two Technologies to Four African Nations
Read More
Department of Telecommunications R&D Centre C-DOT and IIT Delhi Sign MoU for Cooperation in Various Emerging Areas of Telecom
Read More
Workshop on Addressing Air Quality Challenges in Delhi-NCT Held at IIT Delhi; Team Sweden, IIT Delhi Identify Possible Areas of Joint Intervention
Read More
Supreme Court Judge Hon’ble Justice D.Y. Chandrachud to Deliver Inaugural Talk for the Office of Diversity and Inclusion at IIT Delhi
Read More
Hon’ble Union Education Minister, Shri Dharmendra Pradhan Addresses IIT Delhi Diamond Jubilee Celebrations Closing Ceremony
Read More
IIT Delhi Diamond Jubilee Celebrations Closing Ceremony- Director Prof. Rangan Banerjee's Speech
Read More
IIT Delhi Diamond Jubilee Celebrations Closing Ceremony- Hon'ble President of India, Smt. Droupadi Murmu's Speech
Read More
Hon’ble President of India, Smt. Droupadi Murmu Graces IIT Delhi Diamond Jubilee Celebrations Closing Ceremony
Read More
FSAE Italy 2022- IIT Delhi’s Automobile Club AXLR8R Formula Racing Secures First Position in Cost and Manufacturing Event
Read More
Union Minister Shri Piyush Goyal Inaugurates Public Systems Lab at IIT Delhi Established in Partnership with UNWFP
Read More
IIT Delhi’s Technology Innovation Hub IHFC Gets 12 Projects Under DST-NSF Joint Research and Development Program
Read More_1659327288.jpg)
IIT Delhi Organises Academic Outreach Day for Students and Faculty of Haryana Govt. Technical Universities and Colleges
Read More
NIRF India Rankings 2022- IIT Delhi Achieves 2nd Rank in Engineering; Jumps to 4th in Management; Features Among Top 3 Research Institutes
Read More
Empanelment of retired officers as an Inquiry Officers for conducting Departmental Inquiry - reg.
Read More
First Batch Successfully Completes IIT Delhi’s STEM Mentorship Program for High Schoolgirls
Read More
School students showcase promising prototypes they built at Change.Makers summer boot camp organised by IIT Delhi
Read More
IITs in Delhi, Bombay and Kanpur Offer Internship and Sponsored M. Tech. Programmes to Students from Ladakh
Read More
IHFC, TIH of IIT Delhi, Celebrates its 2nd Anniversary; Announces Call for Proposal in Areas of Autonomous Vehicles, Nano Robotics, Block Chain for Applications in Robotics
Read More
Samsung Launches ‘Solve for Tomorrow’, an Innovation Contest for India’s Youth to Crack Real-World Problems; FITT at IIT Delhi to be the Knowledge Partner
Read More
QS World University Rankings 2023- IIT Delhi Achieves an Improved Overall Rank of 174 Globally
Read More
DEPARTMENT OF HUMANITIES AND SOCIAL SCIENCES, IIT DELHI --- Indian & Foreign Languages Learning Programme
Read More
Result- UG - Summer Research Fellowship Programme - 2022 ( IN CONTINUATION TO THE LIST OF STUDENTS PUBLISHED on 13th May, 2022 )
Read More
Technology Innovation Hub of IIT Delhi (IHFC) Signs MoU with the Council for the Indian School Certificate Examinations (CISCE)
Read More
Did Climate Change Cause Ancient Civilizations to Collapse? 08thIIT Delhi SciTech Spins Lecture to Explain
Read More
PG Admission including Ph.D. - Last date for submission of online application and application fee has been extended to April 17, 2022 (4 pm). No further extension will be made.
Read More
QS World University Rankings by Subject 2022 - Five IIT Delhi Academic Programmes in Top 100
Read More
IIT Delhi, Delhi Jal Board Sign MoU to Address Water Security Issues Faced by NCT of Delhi
Read More
World Class Indoor Sports Complex Built with Alumnus Saurabh Mittal's Support Inaugurated at IIT Delhi
Read More
2nd online CEP Certificate programme titled Executive Management Programme in Entrepreneurship Development (EMPED)
Read More
Fabiosys Innovations, an IIT Delhi Startup, Develops Technology to Manufacture Extremely Affordable and Highly Effective Antiviral Fabric
Read MoreCOVID-related Research & Development Work by Centrally Funded Technical Institutes (CFTIs), Ministry of Education, Government of India - February 2022
Read More
IHFC, Technology Innovation Hub of IIT Delhi, Collaborates with US’ National Science Foundation for Research in Cobotics, AI
Read More
Webinar on "What's next in computing: when classical and quantum computing meet" by Dr. Mukesh V. Khare, Vice President, Semiconductor & Cloud, IBM Research, USA on March 04 (Friday) 2022, 8:30 AM IST
Read More
HORIBA India, IIT Delhi Join Hands to Establish Research Center at the Institute’s Chemistry Department
Read More
Troop Comforts Ltd Signs MoU with IIT Delhi to Develop Smart Protective Clothing for Indian Security Forces
Read More
SciTech Spins Lecture: School Students to Learn About Role of Imaging in Advancing Science and Technology
Read More
IIT Delhi Launches an Interactive Website of IIT-PAL to Help High School Students Prepare for Competitive Exams
Read More
IIT Delhi Researchers Develop High Efficiency, Shadow-less, Portable Solar PV Towers for Power Generation
Read More
IIT Delhi Sets up “Pillay Chair Professor” for Research in Machine Learning, VLSI Design and Sensors
Read More
IIT Delhi Students Receive Record Number of Job Offers Up to December 15 of Placement Drive 2021
Read More
SciTech Spins 4th Lecture: School Students to Learn About Mysteries of Universe from IIT Delhi Scientists
Read More
IIT Delhi’s State of the Art Research & Innovation Park Wins Prestigious Façade Project of the Year Award 2021
Read More
Brain Storming Conclave on Atmanirbhar North East through S&T Interventions at Cotton University, Guwahati, Assam December 21–22, 2021
Read More
IntelliSmart & IIT Delhi collaborate to develop next generation Smart Grid technology solutions & capabilities
Read More
IIT Delhi Collaborates with National Law University Delhi for Ushering in Tech-empowered Justice System
Read More
IIT Delhi and Business Sweden - The Swedish Trade and Invest Council Sign MoU for Clean Air and Green Energy Collaborations
Read More
Neilom Prize 2020-21: Recent Graduates of IIT Delhi Awarded for their Work in the Field of Assistive Technology
Read More
‘Why did the Titanic Sink?’ 3rd SciTech Spins Lecture by IIT Delhi to Explain This and Many More Questions to School Students
Read More
Technology Innovation Hubs of IIT Delhi and IIIT Delhi Sign MoU to set up India’s First Medical Cobotics Centre
Read More
State-of-the-art Laboratories Inaugurated at IIT Delhi’s Centre Focusing on Electric Vehicle Technologies
Read More
IIT Delhi, AIIMS New Delhi Jointly Establish Centre for Advanced Research and Excellence in Disability & Assistive Technology (CARE-DAT), a Centre of Excellence
Read More
IIT Delhi’s School of Artificial Intelligence to Start ‘M.Tech in Machine Intelligence & Data Science (MINDS)’
Read More
Applications are invited for appointment to the post of Director, Indian Institute of Technology (llT) Bhubaneswar
Read More
Advertisement inviting applications for the post of Director, IIT Palakkad, IIT Tirupati, IIT Dharwad, IIT Bhilai, IIT Goa and IIT Jammu
Read More
IIT Delhi to Teach School Students ‘How Powerful Computers Can Help in Providing Insights into Real-Life Phenomena’
Read More
IIT Delhi Announces Scholarships and Seed Funding Programs to Mark 2nd Anniversary of its Alumni Endowment Fund
Read More
IIT Delhi Launches New UG Programme ‘B. Tech. in Energy Engineering’; JEE (Advanced) Qualified Students Eligible
Read More
Alumnus Mohit Aron Gifts USD 1 Million to IIT Delhi’s Computer Science and Engineering Department
Read More
B.Tech. Students of NIT Sikkim to be Eligible for Direct Admission to IIT Delhi’s PhD Programmes
Read More
JK Paper Signs MoU with IIT Delhi to Set Up JK Paper Centre of Excellence in Paper and Packaging
Read More
IIT Delhi’s Over Rs 500 Cr State-of-the Art ‘Central Research Facility’ Now Open for Researchers from Across Country
Read More
Optics and Photonics Centre of IIT Delhi launches an outreach initiative named "Optics Learning Centre"
Read More
IIT Delhi Launches Sci-Tech Spins - A Series of Weekend Seminars & Laboratory Demos for High School Students
Read More
DAKSH Centre of Excellence (CoE) for Law & Technology, IIT Delhi Releases Report on Six High Court Websites
Read More
"ComIN21 Asian Voices in Pandemic" conference organized by the Department of Design, IIT Delhi in December 2021
Read More
Seminar on Benefits and Challenges in the Next Decade of Semiconductor Innovation by Dr. Randhir Thakur, Senior Vice President, Intel, USA on 19.08.2021 at 10:00 am
Read More
Unnat Bharat Abhiyan Announces Results of Regional Level Poster and Video Competition for COVID-19 Awareness
Read More
Vipula and Mahesh Chaturvedi Foundation Signs MoU to Attract Outstanding Talent to IIT Delhi
Read More
An Institute Lecture on "Ways to a Carbon-free world" by Mr. Sumant Sinha, Chairman and Managing Director, ReNew Power
Read More
National Health Authority and Indian Institute of Technology Delhi join hands to scale high-potential healthcare innovations
Read More
Attention 2020 Entry UG students: Final list of UG students (2020 Entry) for Change of Programme at the end of 1st Year
Read More
IIT Delhi Establishes Chairs to Support Research in Microelectronics & VLSI Design and Geotechnical & Geo-Environmental Engineering
Read More
IFFCO Signs MoU with IIT Delhi for Innovative & Collaborative Projects to Bring Labs to Farms
Read More
IIT Delhi Alumni Endow Indu Shrivastava & Serla Singh Chair Professor in Artificial Intelligence
Read More
Covid-19: What the pandemic has taught us and the way forward lecture by Prof./Dr. Randeep Guleria, Director, All India Institute of Medical Science (AIIMS), Delhi
Read More
IIT Delhi Establishes Manish Singhal Chair to Promote Teaching and Research in the Area of Smart Textiles
Read More
Supreme Court Judge Mr. Justice S. Ravindra Bhat inaugurates UJF Lab Facility on AI for Judiciary at IIT Delhi
Read More
PAID COVID VACCINATION CAMP - COVISHIELD - 8TH JUNE, 2021 (Tuesday), L.H.C., IIT CAMPUS - EMPLOYEES (FACULTY & STAFF)
Read More
IIT Delhi to Establish Department of Energy Science and Engineering: New UG Programme B.Tech. in Energy Engineering To Be Offered from This Year
Read MoreCovid-19: IIT Delhi collaborates with Delhi Government to improve oxygen infrastructure and supply chain management in Delhi
Read More
IIT Delhi to Create New Centre to Synergize and Boost R&D Activities in Optics and Photonics Field
Read More


































































































_1731498114.jpg)





























































































































_1691059736.jpg)



























































































_1651059243.png)