Publish Date: August 21, 2024
IIT Delhi Researchers Find a Potential Solution to Regulate Dendrite Growth in Room-Temperature Sodium-Sulfur Batteries
Share this on
IIT Delhi Researchers Find a Potential Solution to Regulate Dendrite Growth in Room-Temperature Sodium-Sulfur Batteries
- Study paves the path for the development of an alternative to lithium-ion batteries
New Delhi: The scarcity of electrode materials and the supply chain of other critical components has challenged the sustainability of lithium-ion batteries, prompting battery manufacturers to look for an alternative battery technology.
The room-temperature sodium-sulfur (RT-Na/S) batteries, which comprise abundant and inexpensive electrode materials in the form of sodium and sulfur and rely on a different kind of chemical reaction, making them capable of storing much more energy in comparison to state-of-the-art lithium-ion batteries, may prove a potential alternative.
However, one of the main problems with RT-Na/S batteries has been dendrite growth on the sodium metal anode, causing the cell to fail prematurely.
The dendrites are the branched structures that inherently develop on most metal anodes. There are two causes for the dendrite growth: first, the electrolyte constantly reacts with the sodium metal anode, causing the local surface to be highly irregular. Secondly, the solid electrolyte interphase, a thin, electrically insulating but ionically conducting region, is mechanically and chemically unstable.
Researchers at the Department of Energy Science and Engineering (DESE), IIT Delhi, working to find a possible solution to this dendrite-related problem, have managed to stabilize room-temperature sodium-sulfur battery technology by employing an iodide-based additive in the organic electrolyte solution.
Prof. Vipin Kumar and research scholar Chhail Bihari employed Bismuth iodide (BiI3) as an additive molecule to alter the properties of the electrolyte. BiI3 reduces the energy required for sodium ions to leave the solvent and enter the electrode, improving the charge transfer kinetics. This results in better battery efficiency and faster charging times.

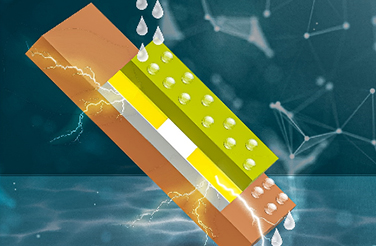
Fig. 1. Schematic illustration of the RT-Na/S battery with and without additives
Moreover, the presence of BiI3 contributes to the formation of a Na3Bi alloy interphase and stable solid electrolyte interphase (SEI) on the sodium metal anode. This layer is crucial as it prevents the growth of sodium dendrites, which can cause short circuits and degrade the battery's performance over time (Fig. 1).
The test-cell prototypes have demonstrated surviving at least 250 charge-discharge cycles while maintaining a much larger capacity than lithium-ion batteries of the same weight.
“This exciting development highlights the importance of innovative research in addressing global energy challenges. By harnessing the power of abundant and safe materials like sodium and sulfur and enhancing their performance with innovative additives like BiI3, we move closer to a future where sustainable energy storage is accessible to all. Room-temperature sodium-sulfur batteries would be ideal for use in electric vehicles and grid applications,” said Prof. Vipin Kumar from the Department of Energy Science and Engineering, IIT Delhi.
The findings of the study were recently published in the Journal of Materials Chemistry A- https://pubs.rsc.org/en/content/articlelanding/2024/ta/d4ta03187c
ENDS
(PRESS RELEASE ISSUED ON AUGUST 16, 2024)