Publish Date: July 9, 2024
Peptide Catalyzes New Molecular Synthesis with Unprecedented Precision and Selectivity
Share this on
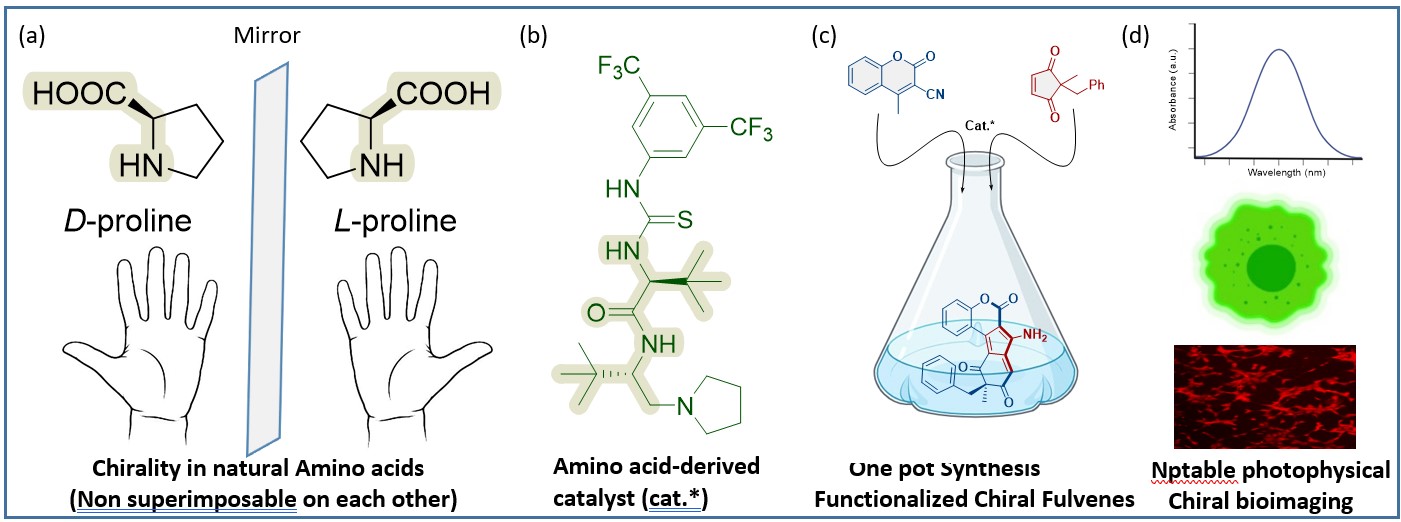
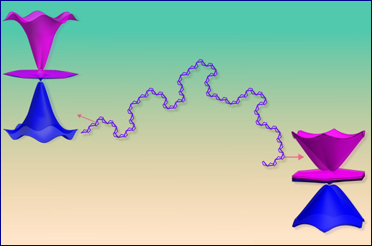
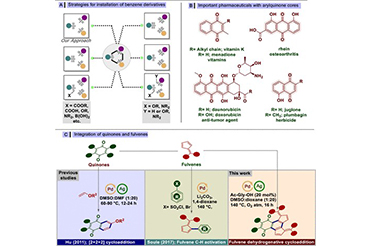
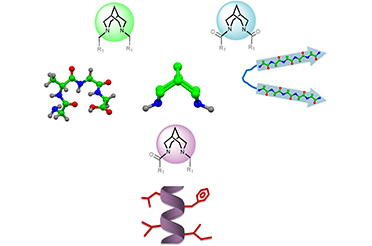
(Single step-one-pot synthesis of small molecules, containing chiral amino-fulvenes, with excellent pharmacological and bio-imaging properties, catalysed by chiral natural amino acids. Catalysts have the potential to expedite the production of new compounds, such as pharmaceuticals. Credit: IIT Delhi/Ravi P Singh illustration)
Inspired by the nature’s small molecule synthesis prowess’s and with a goal to develop a green sustainable synthetic route towards medicinally important small molecules, scientists at IIT Delhi have manipulated commonly available amino acids to design an organic catalyst for achieving a variety of chiral small molecules without use of toxic, unnatural metal catalysts. The organocatalysts developed from amino acid t-Leucine, can carry out reactions that can yield elusive chiral fulvenes in a single step.
The researchers led by Prof. Ravi P. Singh, Department of Chemistry, IIT Delhi, were inspired by enzymes especially, the dipeptides, which are known to facilitate chemical process with ease in the absence of metal ions. Since catalysis frequently uses transition metals, which are conductive metals such as titanium, iron, nickel, silver, copper, palladium, and gold, they are often considered quite toxic to the environment and search for greener methods is an ever-challenging problem.
According to Prof. Singh, the new catalysts and synthetic procedure described in the Nature Commun 15, 2101 (2024) opens options for quicker and more affordable chemical synthesis, especially of a scaffold such as Fulvene, which is of huge significance in biology and medicine. Since Fulvenes are aromatic compounds, these become even more diverse and potent when functionalized, or changed, and combined with a chiral center. Unfortunately, green, economical and straight forward methods for obtaining its derivatives are quite limited according to Singh.
The method developed yielded new molecules, which were all in single-enantiomers suggesting the high selectivity achieved in product formation. Most biologically active, asymmetrical molecules exist in two forms structurally, called enantiomers. Enantiomers have structures that are mirror images, like one's hands, and cannot be placed upon one another. Notably, when these molecular twins engage with enzymes, proteins, receptors, and even other chiral catalysts, they might have drastically divergent effects, one positive and one negative. Thus, it makes sense that pharmaceutical corporations are all focused towards synthesizing drugs that solely have the beneficial enantiomer. Currently, single-enantiomer compounds are painstakingly synthesized as building blocks for drugs, agrochemicals and functional materials.
“Almost 99 percent of chiral drugs will be sold as single enantiomers. However, it is difficult to precisely and efficiently synthesize a single enantiomer, especially using trial-and-error methods that currently frequently calls on transition metal catalysts. Catalysts are necessary for enantiomer preparations," he stated; however, transition metals apart from being expensive and toxic, must also be completely removed before the molecule is utilized in clinical studies.
"We have been developing organocatalysts for almost a decade and designing economical, industrially viable and environmentally benign protocols for achieving single enantiomers and not racemate molecules is the basis of what we do," Singh stated. “While investigating the different combinations of natural amino acids for synthesizing chiral complex biologically important molecular scaffolds, we discovered this fascinating reaction that unfolds in a cascading manner."
Utilizing easily available chemicals such as cyclopenten 1,3-diketone and coumarins, the team created functionalized aminofulvene, which according to the group is a major advance.
"We quickly synthesized about 49 distinct chiral amino fulvene molecules using corresponding building pieces. The chiral fulvenes that have been further functionalized and are showing incredible bioactivity and optical properties. The group in their report demonstrated the usefulness and importance of enantiomers of the molecules synthesized in cellular labelling as they possessed excellent fluorsecnt properties and were non-cytotoxic making them apt for bio-imaging. Interestingly, the catalysts developed can be used directly as organocatalysts or in combination with transition metals to form new transition-metal catalysts by complexation. Thus, the options are virtually endless. Furthermore, these substances can serve as building blocks for natural molecules and pharmaceuticals that contain fulvene substructures."
The fulvenes, he claimed, "open up an entire world of new chemical space" because they make the process of creating new chemical compounds easier.
"There are a lot of implications," stated Singh. "This will certainly find its way into drug discovery, making agrochemicals and many other fine chemicals."
ENDS