Publish Date: 15th September 2020
FDA Approved Drug Teicoplanin Found Effective Against Main Protease of COVID -19 virus: Study
Share this on
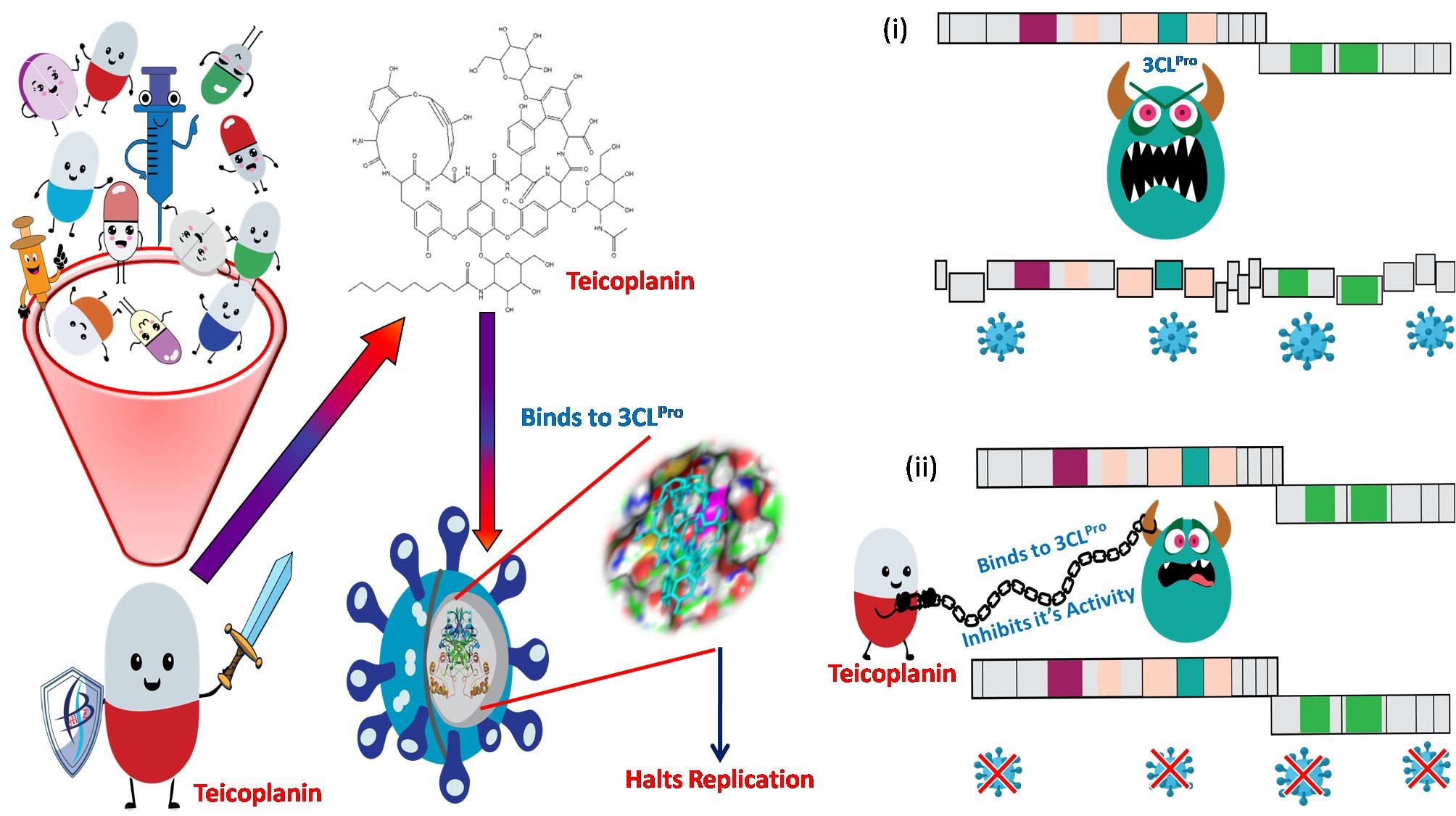
An interesting research study at Kusuma School of Biological Sciences, IIT Delhi, conducted by the research group of Prof. Ashok Kumar Patel, which was recently published in the International Journal of Biological Macromolecules (Impact Factor-5.162) has proposed the clinically approved drug Teicoplanin as a potential therapeutic option against SARS-CoV-2.
This study screened an assemblage of 23 approved drugs, which have shown leads towards being therapeutic options for COVID-19 for those having an inhibitory effect towards the main protease of the virus 3CLPro and among them, the drug Teicoplanin showed the most promising inhibition of the proteolytic activity of this main viral protease. The 3CLPro protease (3-chymotrypsin-like protease), also called the main protease of the virus, is necessary for processing the viral polyproteins and therefore has emerged as an exciting premise for the development of drugs targeting the virus.
Prof. Ashok Patel, KSBS, IIT Delhi said, “While the effect of Teicoplanin was compared with other important drugs in use, Teicoplanin was found to be 10-20 fold more effective than the chief drugs being used against SARS-CoV-2, such as Lopinavir and Hydroxychloroquine in our laboratory conditions. Teicoplanin is an FDA-approved glycopeptide antibiotic, which is regularly used for treating Gram-positive bacterial infections with low toxicity profile in humans."
The research group led by Prof. Ashok Patel includes Praveen Kumar Tripathi, Saurabh Upadhyay, Dr. Manju Singh, Dr. Siva Raghavendhar, Mohit Bharadwaj, and Dr. Pradeep Sharma (AIIMS).
Simultaneous study on Teicoplanin by Intensive Care COVID-19 Study Group of Sapienza University
Recently, there has been a clinical study carried out with Teicoplanin as reported by Intensive Care COVID-19 Study Group of Sapienza University. They recruited a cohort of 21 patients affected by severe COVID-19 symptoms such as lung involvement, who were hospitalized in intensive care units (ICUs) of a hospital in Italy, Rome, and complementarily treated with Teicoplanin. On ICU admission, the patients received Teicoplanin in doses of 6 mg/kg every 24 h. The median duration of Teicoplanin therapy was 10 days (range 7–12 days). The ICU mortality rate was only 14.3% (3/21 patients). None of the patients had any adverse effects related to Teicoplanin.
In a nutshell, all studies support each other that Teicoplanin might be a potential therapeutic option against COVID-19.
However, a more detailed clinical investigation is required on a large cohort, in different stages mild, moderate and critically ill patients to conclude the definite role of Teicoplanin against COVID-19.